Abstract
Background
Outcome of diffuse large B cell lymphoma (DLBCL) with primary treatment failure is variable depending on pattern of failure (PTF-primary progression, <CR or early relapse), NCCN-IPI at time of failure and biological characteristics, particularly the presence of MYC translocations. The ideal salvage therapy for these patients is unknown. The CORAL study did not show significant difference between the combination of rituximab ifosfamide, carboplatin and etoposide (R-ICE) and the combination of rituximab, dexamethasone, high-dose cytarabine and cisplatin (R-DHAP) in patients with refractory or relapsed DLBCL. However, a post hoc analysis indicated that patients with germinal center B (GCB)-type DLBCL may have better outcomes with R-DHAP. We utilized a retrospective dataset of DLBCL patients to primary treatment failure to exam potential outcomes differences according to putative cell of origin (COO).
Methods
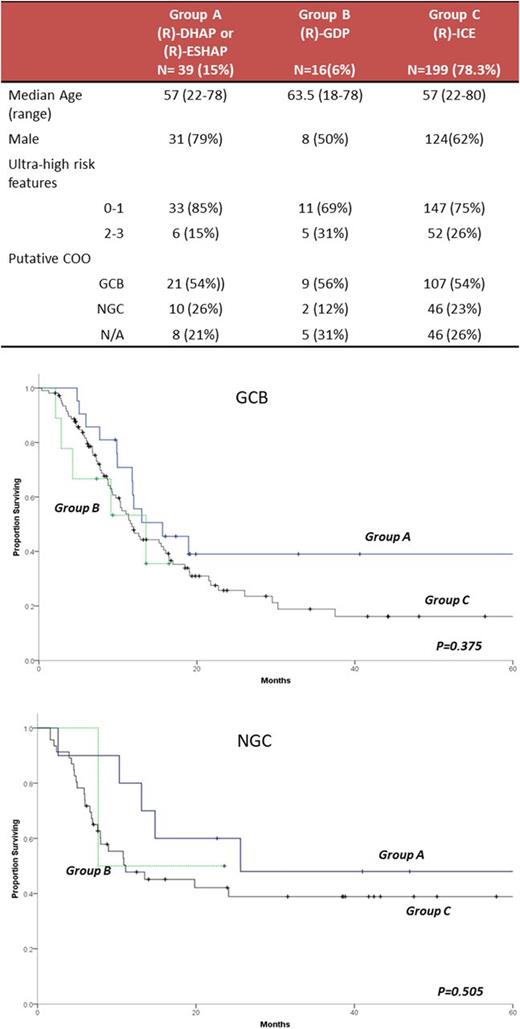
Patients evaluated were the subset of patients participated in the retrospective, multicenter REFINE (Registry of DLBCL with PTF) study. We included 254 patients with DLBCL and PTF excluding those with primary CNS lymphoma; HIV associated lymphoma and transformed DLBCL and who received a platinum-based salvage regimen. Patients were classified in three groups according to first salvage chemotherapy regimen. Patients in group A received a high-dose cytarabine and cisplatin containing regimen, either (R)-DHAP or the combination of etoposide, methylprednisolone, high-dose cytarbine and cisplatin with or without rituximab [(R)-ESHAP]. Patients in group B received a gemcitabine, dexamethasone and cisplatin combination [(R)-GDP] and patients in group C received (R)-ICE. We performed univariate comparison between the three groups in terms of overall response to salvage therapy (ORR=PR+CR) and overall survival (OS) and multivariate analysis for survival, adjusting the presence of ultra-high risk (UHR) features: Progression on first line therapy, MYC translocation and NCCN-IPI intermediate high/high risk.
Results:
Patients' characteristics are displayed in the Table. Overall there was no difference in ORR for patients in groups A, B or C (49% vs. 38% vs. 47%, P=0.730). Among patients with GCB DLBCL, responses were seen in 11/21 (52%) vs. 3/9 (33%) vs. 49/107(46%), P=0.629 while in NGC DLBCL, responses were seen in 6/10 (60%) vs. 2/2 (100%) vs. 21/46 (46%), P=0.253, in groups A, B and C respectively. Among GCB DLBCL patients, there was no difference in proportion of patients reaching hematopoietic cell transplantation (HCT) across the 3 treatment groups (38.1% vs. 33.3% vs. 43.9% for groups A, B and C respectively). Among NGC DLBCL a higher proportion of patients in group A (9/10=90%) and B (2/2=100%) than in group C (22/49=48%) reached HCT (P=0.023). There was no OS difference between the treatment groups for either GCB and NGC patients (Figure). After adjustment for the presence of UHR features, survival of GCB DLBCL patients in treatment group A (HR 0.818, 95% C.I. 0.440-1.520, P=0.525) or group B (HR 0.896, 95% C.I. 0.344-2.329, P=0.821) was not distinct from survival in group C. Similarly, among NGC DLBCL patients, survival in group A (HR 0.585, 95% C.I. 0.213-1.502, P=0.253) and in group B (HR 0.820, 95% C.I. 0.108-6.231, P=0.848) was comparable to group C.
Conclusion:
In this high risk DLBCL population with PTF we found no indication of superior performance for any of the commonly used platinum-based combination chemotherapy regimens, including within the subset of patients with GCB DLBCL. Treatment outcomes for this population overall are poor and innovative therapeutic strategies are urgently needed.
Hamadani: Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; Celgene, Cellerant, Jansen, MedImmune: Consultancy. Bachanova: Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle-Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Zymogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oxis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees. Maddocks: Merck: Research Funding; BMS: Research Funding; Pharmacylics: Research Funding; Novartis: Research Funding. Chavez: Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Speakers Bureau; Janssen: Speakers Bureau; Kite: Speakers Bureau. Reddy: Gilead: Speakers Bureau; Celgene: Consultancy; BMS: Consultancy; Abbvie: Consultancy. Flowers: Janssen Pharmaceutical: Research Funding; National Institutes Of Health: Research Funding; Millennium/Takeda: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; National Cancer Institute: Research Funding; OptumRx: Consultancy; Acerta: Research Funding; Clinical Care Options: Research Funding; Educational Concepts: Research Funding; Onyx: Research Funding; Spectrum: Consultancy; TG Therapeutics: Research Funding; Infinity: Research Funding; Prime Oncology: Research Funding; Bayer: Consultancy; Celgene: Consultancy, Research Funding; Burroughs Welcome Fund: Research Funding; Genentech/Roche: Consultancy, Research Funding; V Foundation: Research Funding; Seattle Genetics: Consultancy; Gilead: Consultancy; Research to Practice: Research Funding; Abbvie: Consultancy, Research Funding. Evens: Pharmacyclics: Consultancy; Celgene: Consultancy; Millennium: Consultancy; Seattle Genetics: Consultancy; Merck: Consultancy; AbbVie: Consultancy; Kite Pharma: Consultancy; Amgen: Consultancy; Affimed: Consultancy; Novartis: Consultancy; • Spectrum Pharmaceuticals: Consultancy. Lansigan: Spectrum Pharmaceuticals: Consultancy, Research Funding; Seattle Genetics: Consultancy. Cohen: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takada: Research Funding; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; LAM Therapeutics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal